 Diffuse low-grade gliomas
Diffuse low-grade gliomas
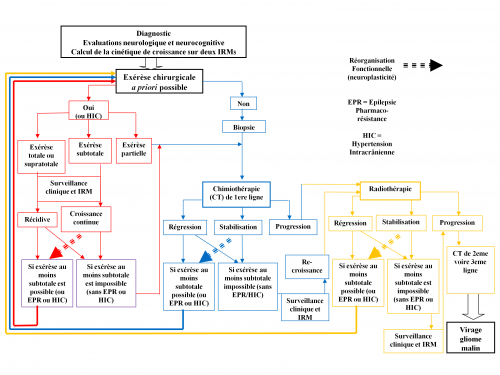
VI - TREATMENT
*1. Therapeutic strategy
The demonstration of the existence of a functional and vital prognosis in less than a decade in young patients leading an active lifestyle at diagnosis, coupled with methodological advances which have enabled a minimization of therapeutic risk, resulted in an early intervention for DLGGs.
Several symptomatic (AEDs) and oncologic treatments (surgical resection, possibly repeated, chemotherapy, radiotherapy) are thus to be considered not in isolation, but in combination, in the view of adapting individualized treatment strategies, and also for each patient at the different phases of disease history.
The purpose of such an attitude is twofold: the preservation and even the improvement of the quality of life and an increased survival time - delaying malignant transformation (26).
*2. Anti-Epileptic Treatment
The management of epilepsy in DLGG has been the subject of recent reviews (62,71). In patients who have had a single attack, immediate antiepileptic treatment reduces the risk of a possible second attack compared to delayed treatment, without major complications with no additional burden on the quality of life.
Older antiepileptic medications, including carbamazepine, phenytoin and valproate have demonstrated their effectiveness against placebo in controlled studies. New medications, including gabapentin, lacosamide, lamotrigine, oxcarbazepine, or topiramate have shown their equivalence but not their superiority compared to carbamazepine, phenytoin and valproate. A randomized trial comparing carbamazepine to the most recent drugs (lamotrigine, gabapentin, oxcarbazepine, or topiramate) showed a longer duration to treatment failure with lamotrigine. Moreover, better cognitive status was also found with lamotrigine.
Levetiracetam, indicated as monotherapy in partial epilepsy, has also demonstrated its equivalence to carbamazepine in a prospective randomized trial.
Levetiracetam may be introduced and increased rapidly, which is an advantage compared to other medications, especially lamotrigine.
It is also available as an injection (eg valproate) with an excellent efficacy / safety ratio in neuro-oncology.
Lacosamide in combination with another treatment appears to be effective and well tolerated.
The enzyme-inducing effect of some antiepileptic medications can lead to an interaction with many chemotherapy drugs (nitrosoureas, paclitaxel, cyclophosphamide, topotecan, irinotecan, thiotepa). Valproate may potentiate the hematologic toxicity associated with chemotherapy.
The decision to possibly stop antiepileptic therapy should be made on a case by case basis depending on the lifestyle of each patient, and must be tapered over a period of about 3 to 6 months
*3. Surgery
Impact of surgical resection on survival in DLGG
Surgery is required in all cases to obtain a tissue sample, in order to differentiate tumor subtypes, to define tumor grade and to study its genetics.
Beyond this histo-molecular goal, the aim of surgery is to strive for a maximal resection in order to minimize the risk of malignant transformation and therefore increase median survival while maintaining or even improving the quality of life of these patients.
To that effect, the extent of resection should always be objectified by post-operative MRI. Indeed, a critical point is the precise definition of complete resection of DLGG which is the total ablation all hyperintense regions on FLAIR sequence, verifiable by comparing pre-and post-surgical MRIs. A subtotal resection is considered with a residual volume of less than 10cc and partial if it is more than 10cc. Despite the absence of randomized trials, all surgical series based on an objective assessment of postoperative MRI have shown a significant impact of the complete or subtotal resection on overall survival in delaying malignant transformation (2,8,9,30, 37,45,65,69).
Specifically, a recent study compared survival in two cohorts of patients with DLGG. In the first group, patients received a single biopsy and follow-up (biopsy and watchful waiting), with a median survival of only 5.9 years. In the second group, early surgical resection was performed: median survival was not reached in the same period, demonstrating the significant impact of the intervention (38). Thus the largest surgical series about 1097 patients has been reported by the French Network for gliomas Study (REG= Réseau d’étude français des gliomes), showing a median survival of 13 years from the first treatment and 15 years from the first symptom - either overall double when compared to watchful waiting (8).
This is why currently a prospective randomized study would not be ethical. Therefore, due to an increased risk of malignant transformation, lower quality of resection due to progression of tumor infiltration during the follow up, early surgery at diagnosis is currently recommended (once there is evidence of volumetric increase) (21,64). Biopsy (while conscious of the risk of tumor under grading aforementioned) should be reserved exclusively when intervention is contraindicated, particularly in gliomatosis. Surgery for asymptomatic DLGG is now being discussed, as it results in a greater number of total resections due to smaller tumor volume (24).
In conclusion, according to current European recommendations surgical resection represents the first therapeutic option in DLGG (72).
Functional consideration
A recent meta-analysis showed beyond the fact that although the use of intra-operative electrical stimulation mapping techniques significantly increased the percentage of patients with total or subtotal resection, they significantly decreased the rate of permanent postoperative deficits (17).
In this sense, awake surgery is a well tolerated procedure (see Chapter by Zemmoura and Duffau for technical aspects), which allows (i) to expand the indications for surgery in ’eloquent’ areas (3) (ii) to identify critical functional cortical and subcortical structures, especially related to sensorimotor, language, visual-spatial, cognitive and emotional processing (iii) to reduce the risk of permanent neurological sequelae to less than 2% (29.63 ) (iv) to perform resection according to functional boundaries without margins (rather than on purely anatomical or ’oncologic” borders ) which gives optimization of the extent of resection (14) (v) and to increase overall survival (23) .
In fact, a recent study showed that the use of functional mapping in awake patients at high risk because of the localization of DLGG in eloquent areas resulted in very significant increase in the long-term survival through an increase in the extent of resection (11).
Moreover, awake surgery in ’non-functional’ areas can lead to a ’supra-total’ resection – that is, to remove a safety margin around the FLAIR hyperintensity visible on MRI as tumor cells infiltrate within 1 to 2 cm (54), with a very significant impact on the malignant transformation (77). When total resection is not feasible for functional reasons, one or more re-do interventions can be considered, with an impact on overall survival while preserving brain function (8.50).
Indeed, awake surgery has equally enabled the performance of one or more reoperations with functional preservation and improvement in the extent of resection, including eloquent areas, through mechanisms of brain plasticity (20) (Figure 2).
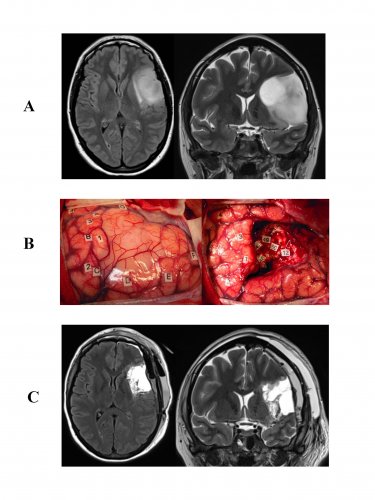
Resection of a DLGG infiltrating the left opercular region ("Broca’s area") with invasion of the insula.
A: FLAIR MRI sequences, axial (left) and coronal T2 (right) showing a typical image of a left operculo-insular DLGG in a right-handed 35 year old patient who experienced an inaugural seizure. Neurological examination was normal preoperatively, but the neuropsychological assessment objectified disorders verbal working memory.
B: Intraoperative photographs before (left) and after (right) surgical resection performed according to cortical and subcortical intraoperative functional boundaries in an awake patient. The letters symbolize the tumor boundaries identified through an intraoperative ultrasound system. The numbers correspond to "eloquent" areas as follows:![]() Cortical areas 1 and 3 (speech arrest at the ventral premotor cortex); 2: primary motor area of the face; 4: anomic area (posterior superior temporal gyrus);
Cortical areas 1 and 3 (speech arrest at the ventral premotor cortex); 2: primary motor area of the face; 4: anomic area (posterior superior temporal gyrus);![]() Subcortical areas: 48: anarthria generated by stimulation of the anterior portion of the lateral upper longitudinal tract (ending at the ventral premotor cortex); 49, 46, 47: semantic paraphasias induced by stimulation of the inferior fronto-occipital tract (courses in the temporal stem and frontal lobe, terminating at the dorsolateral prefrontal cortex); 50: "Negative motor" network resulting in interruption of movement during stimulation and projecting into the anterior limb of the internal capsule; 12: perseverations generated by stimulation of the head of the caudate nucleus.
Subcortical areas: 48: anarthria generated by stimulation of the anterior portion of the lateral upper longitudinal tract (ending at the ventral premotor cortex); 49, 46, 47: semantic paraphasias induced by stimulation of the inferior fronto-occipital tract (courses in the temporal stem and frontal lobe, terminating at the dorsolateral prefrontal cortex); 50: "Negative motor" network resulting in interruption of movement during stimulation and projecting into the anterior limb of the internal capsule; 12: perseverations generated by stimulation of the head of the caudate nucleus.
C: FLAIR sequences: axial (left) and coronal T2 (right) on early postoperative MRI (done six hours after surgery), demonstrating complete resection. The diagnosis of WHO grade II glioma was confirmed histologically. After a transient worsening in speech requiring speech therapy a few weeks at home, the patient resumed a normal social and professional life - with improved neuropsychological assessment performed 3 months after surgery in comparison to the preoperative assessment. No adjuvant cancer treatment was given, but clinical follow up and regular MRI was introduced.
Moreover, wide resection, especially if complete on imaging, improve epilepsy control in approximately 80% of cases, particularly in patients with prolonged preoperative epilepsy and in cases of insular gliomas (33).
Finally, with an individualized functional and cognitive rehabilitation in the early postoperative period, neuropsychological improvements have even been shown in 30% of patients following resection - especially working memory (73). It must be emphasized that a recent randomized trial showed that cognitive rehabilitation had a positive impact on cognitive symptoms in the short and long-term as well as mental fatigue among patients with glioma patients (31).
In summary, early more radical surgery as possible, should be considered as first-line therapy in DLGG because of the favorable impact on both survival and quality of life.
*4. Chemotherapy
The usefulness of chemotherapy in operated and irradiated patients has been well established, especially for oligodendrogliomas. The combination Procarbazine, CCNU and vincristine (PCV) as well as Temozolomide resulted in 45-62% response rate at 10-24 months duration objectified on relatively similar imaging- albeit with a less toxicity and better tolerance on Temozolomide (58).
In addition, a functional benefit, particularly due to a favorable impact on seizures, is frequently observed not only in patients with significant radiological regression but also in tumors stabilized by chemotherapy.
Chemotherapy with PCV or Temozolomide has also been administered postoperatively before radiotherapy, particularly in patients with partial resection, intractable epilepsy and / or rapid progression on imaging control. The majority of patients were classified as ’responders’ most of the time with a stabilization or volumic regression, although usually partial. This effect may be delayed until 24-30 months and may persist despite discontinuation of chemotherapy (59).
Even though the chances of response are greater for oligodendroglial tumors, they are not insignificant for astrocyte or mixed tumors.
Most patients with drug-resistant epilepsy may benefit from a reduction in the frequency and intensity of crises, including in the absence of volume regression on MRI.
It is worth reemphasizing that the conventional MacDonald criteria radiological are not suitable for monitoring patients treated for DLGG, especially in the absence of contrast enhancement, and evolutionary kinetic calculations should be made from now on the basis MRI T2 / FLAIR sequences after volumic measurements (55).
Neoadjuvant chemotherapy may also be given as first line treatment in inoperable tumors due to massive infiltration of functional zones. This chemotherapy may allow regression of infiltration and open the door to a second surgical resection while preserving the quality of life (5).
Although the impact of 1p19q deletion on the response rate to chemotherapy remains a subject of controversy, response duration seems longer in case of co-deletion
The lowest doses of Temozolomide administered daily 3 weeks out of 4 could provide an advantage compared to the usual doses given 5 days per month, especially in unmethylated gliomas, but at the cost of a possible increase in toxicity.
Quality of life does seem generally unaffected by taking Temozolomide for months or years (5).
*5. Radiotherapy
The role of radiotherapy in the treatment of DLGG has been completely revised in recent years; while early irradiation has long been advocated, irradiation should now be postponed. Indeed, a prospective randomized study (EORTC 22845) comparing early versus delayed radiotherapy showed that although progression-free survival was increased during early radiotherapy, no significant difference was eventually found on overall survival because of the time of irradiation (74).
Furthermore, patients treated with whole-brain irradiation have seen a higher incidence of leukoencephalopathy and cognitive deficits compared to those with focal radiotherapy. A recent study also demonstrated in patients with a neuropsychological follow-up of 12 years without tumor recurrence that those without irradiation had preserved cognitive function while those irradiated showed a worsening of attention and executive functions as well as a slowdown in information processing speed in 57% of cases (18).
A multivariate analysis favored a predominant involvement of attention functions. In addition, two randomized trials have studied different doses of irradiation: the EORTC study (45 versus 59.4 Gy) and NCCTG (50.4 versus 64.8 Gy) showed no benefit of high-dose compared with lower doses (39, 67). On the contrary, increased toxicity was induced by higher doses, with an incidence of radiation necrosis of 2.5% within 2 years post-radiotherapy or a negative impact on the quality of life, particularly as a result of greater fatigue, insomnia and emotional disorders.
Therefore, radiation therapy is currently used only in case of treatment failure after surgery (s) and chemotherapy (s), at a lower dose and focally - except in certain cases of intractable epilepsy, since irradiation could allow control of the latter (62).
Finally, it is important to stress that the RTOG 9802 trial compared radiotherapy alone versus radiotherapy with PCV chemotherapy (68). In the radiotherapy alone arm, since two-thirds of patients had received chemotherapy due to secondary progression, thus finally, the study can be regarded as a comparison of early chemotherapy versus chemotherapy on tumor progression. Progression-free survival was improved, but not overall survival. However, beyond 2 years, the addition of PCV chemotherapy to radiation reduced the risk of death by 48% and the risk of progression by 55%, suggesting a delayed effect of chemotherapy. NCI grade 3-4 toxicity was however higher in patients treated with radiotherapy and chemotherapy (67% versus 9%).
*6. Towards an individualized and serial therapeutic strategy
The optimal management needs to be tailored according to the complex biological behavior of each DLGG. In classical literature, the vast majority of studies have investigated the role of a single treatment in isolation (role of surgery, chemotherapy or radiotherapy) without an overall vision of the entire strategy.
The goal now is to evolve towards a holistic approach, based on the anticipation of an individualized and long-term multi-therapeutic approach while adapting to real-time strategy over the years by referring to the clinical, radiological, and histo-molecular updates made possible through regular monitoring ad vitam aeternam.
Such a dynamic attitude challenges the usual (traditional) care in many ways: by proposing early treatment, repeated therapy (e.g. 2 to 4 surgical resection several years apart, or periods of chemotherapy intercalated by simple monitoring, etc.); modifying ’classical order’ of treatments (e.g., preoperative neoadjuvant chemotherapy followed by surgery, no early radiotherapy, etc); and all aiming at ultimate optimization of both median survival and quality of life.
To that effect, beyond the risk / benefit ratio of each treatment considered alone, the impact of the global therapeutic strategy on the cumulative time of optimal quality of life versus time to malignant transformation should be systematically taken into account - not just survival regardless of the functional status of the patient. In other words, the individualized management should be based on understanding the interactions between the natural history of DLGG, mechanisms of reactionary neuroplasticity and onco-functional modulation induced by multiple therapies (Figure 3) (21 , 26).
 Encyclopædia Neurochirurgica
Encyclopædia Neurochirurgica